| Availability: | |
|---|---|
| Quantity: | |
DINGMIAO
Product Video
Product Show
The advantages of Botulax is that Botulax is strictly controlled for safety and high stable potency, has purity of 99%. Botulax contains 100 units (U) of Clostridium botulinum toxin type A, 0.5 milligrams of albumin (human)and 0.9 milligrams of sodium chloride in a sterile, vacuum-dried from without a preservative.
It appears as a lyophilized white powder for injection in a colorless transparent vial.
Product Usage
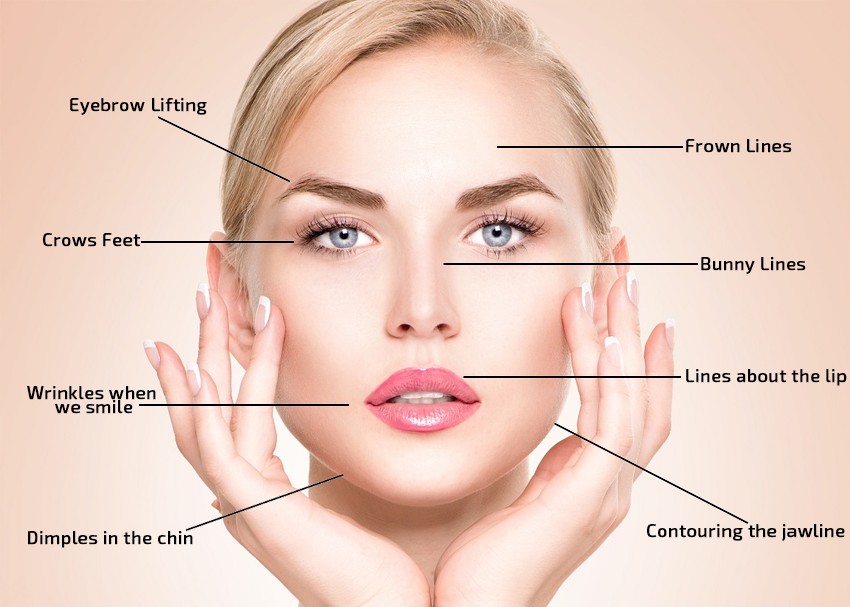
Botulax 100 (Type A) is a cosmetic injection that blocks the nerve signals that cause muscles to contract. This effect relaxes and smooths the look of lines and wrinkles caused by repetitive movements on the face-most commonly, between the brows, crows-feet around the eyes, and horizontal forehead creases. It is also used cosmetically to balance facial asymmetry and relax tight neck bands, as well as medically to reduce perspiration and to treat migraine headaches and muscle spasticity.
Product Advantages
Resume normal work right away
Just by few tiny injections
Effects visible after one administration, starting from 2 weeks after injection, and peaks around 3~4 weeks.
Painless even when used without aenesthetic
Effects last for 6~12 months
Approved by Health Authorities with over 8 years’ clinical practices.
Rug Effects
After Injection
There is the possibility of reactions at the site of an injection, and effects beyond the injection site, including but not limited to asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. Serious hypersensitivity has been reported as rare.
There is a caution for people with pre-existing neuromuscular disorders, and for those with corneal exposure and ulceration in blepharospasm.
Botulium Toxin Type A is not to be administered at all if a patient has a known allergy to any of the ingredients, or if the patients have neuromuscular junctional disorders or known to have adverse reactions.
Other Products
 Hipretty PDO Thread |  Younsofill Dermal Fillers |
 Cindella + Luthione + VitaminC |  Lipolab |  LIporase |
Product Video
Product Show
The advantages of Botulax is that Botulax is strictly controlled for safety and high stable potency, has purity of 99%. Botulax contains 100 units (U) of Clostridium botulinum toxin type A, 0.5 milligrams of albumin (human)and 0.9 milligrams of sodium chloride in a sterile, vacuum-dried from without a preservative.
It appears as a lyophilized white powder for injection in a colorless transparent vial.
Product Usage

Botulax 100 (Type A) is a cosmetic injection that blocks the nerve signals that cause muscles to contract. This effect relaxes and smooths the look of lines and wrinkles caused by repetitive movements on the face-most commonly, between the brows, crows-feet around the eyes, and horizontal forehead creases. It is also used cosmetically to balance facial asymmetry and relax tight neck bands, as well as medically to reduce perspiration and to treat migraine headaches and muscle spasticity.
Product Advantages
Resume normal work right away
Just by few tiny injections
Effects visible after one administration, starting from 2 weeks after injection, and peaks around 3~4 weeks.
Painless even when used without aenesthetic
Effects last for 6~12 months
Approved by Health Authorities with over 8 years’ clinical practices.
Rug Effects
After Injection
There is the possibility of reactions at the site of an injection, and effects beyond the injection site, including but not limited to asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. Serious hypersensitivity has been reported as rare.
There is a caution for people with pre-existing neuromuscular disorders, and for those with corneal exposure and ulceration in blepharospasm.
Botulium Toxin Type A is not to be administered at all if a patient has a known allergy to any of the ingredients, or if the patients have neuromuscular junctional disorders or known to have adverse reactions.
Other Products
 Hipretty PDO Thread |  Younsofill Dermal Fillers |
 Cindella + Luthione + VitaminC |  Lipolab |  LIporase |