| Availability: | |
|---|---|
| Quantity: | |
DINGMIAO
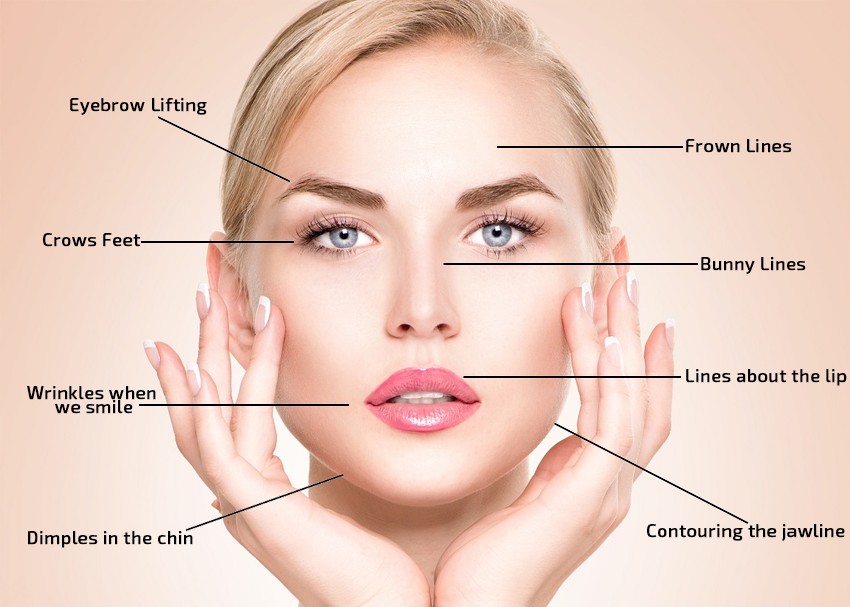
Refresh and Revitalize with Nabota Botulinum Toxin
Experience the future of skincare with Nabota, a trailblazing product for the neuromuscular junction designed to combat wrinkles stemming from facial expressions in the glabella area. Nabota injections, formulated with botulinum toxin type A, offer a secure and highly efficient alternative to traditional Botulinum Toxin . Wave farewell to stubborn frown lines and crow’s feet, and embrace an invigorated, youthful look.
Is This Product For You?
Perfect for people looking for a safe way of eliminating wrinkles from their face and skin.
Ideal for removing lines like expression lines, forehead lines, and frown lines.
For rejuvenating the face, making it smoother and more symmetrical in appearance.
Product Show

| Efficacy Effects | Temporary improvement in the appearance of moderate to severe glabella wrinkles associated with corrugator musleand/or procerus muscle activities |
| Main Ingredients | Clostridium botulinum toxin type A (standard) -100 units |
| Description | White or light yellow lyophilizate in a colorless transparent vial that becomes clear and transparent when dissolved in physiological saline |
| Classification | White or light yellow lyophillizate in a colorless transparent vial that becomes clear and transparent when dissolved in physiological saline |
Product Usage
Ingredient & Content
-In one vial-
100 units of Clostridium botulinum toxin type A (standard), serum albumin (vitality), sodium chloride (USP)
Efficacy Effects
Temporary improvement in the appearance of moderate to severe glabella wrinkles (vertical lines between the eyebrows) associated with corrugator muscle and/or procerus muscle activities, in adults aged between 20 to 65.
Storage Methods
Sealed container, refrigerated (2 ~ 8 C) Storage
Date of Use
36 months from date of manufacture
Product Advantages
Resume normal work right away
Just by few tiny injections
Effects visible after one administration, starting from 2 weeks after injection, and peaks around 3~4 weeks.
Painless even when used without aenesthetic
Effects last for 6~12 months
Approved by Health Authorities with over 8 years’ clinical practices.
Rug Effects
After Injection
There is the possibility of reactions at the site of an injection, and effects beyond the injection site, including but not limited to asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. Serious hypersensitivity has been reported as rare.
There is a caution for people with pre-existing neuromuscular disorders, and for those with corneal exposure and ulceration in blepharospasm.
Botulium Toxin Type A is not to be administered at all if a patient has a known allergy to any of the ingredients, or if the patients have neuromuscular junctional disorders or known to have adverse reactions.
To preserve results achieved after a treatment with this Botulinum Toxin Type A injectable, patients need to undergo maintenance injection procedure
Other Products
 Hipretty PDO Thread |  Younsofill Dermal Fillers |
 Cindella + Luthione + VitaminC |  Lipolab |  LIporase |
Refresh and Revitalize with Nabota Botulinum Toxin
Experience the future of skincare with Nabota, a trailblazing product for the neuromuscular junction designed to combat wrinkles stemming from facial expressions in the glabella area. Nabota injections, formulated with botulinum toxin type A, offer a secure and highly efficient alternative to traditional Botulinum Toxin . Wave farewell to stubborn frown lines and crow’s feet, and embrace an invigorated, youthful look.
Is This Product For You?
Perfect for people looking for a safe way of eliminating wrinkles from their face and skin.
Ideal for removing lines like expression lines, forehead lines, and frown lines.
For rejuvenating the face, making it smoother and more symmetrical in appearance.
Product Show

| Efficacy Effects | Temporary improvement in the appearance of moderate to severe glabella wrinkles associated with corrugator musleand/or procerus muscle activities |
| Main Ingredients | Clostridium botulinum toxin type A (standard) -100 units |
| Description | White or light yellow lyophilizate in a colorless transparent vial that becomes clear and transparent when dissolved in physiological saline |
| Classification | White or light yellow lyophillizate in a colorless transparent vial that becomes clear and transparent when dissolved in physiological saline |
Product Usage

Ingredient & Content
-In one vial-
100 units of Clostridium botulinum toxin type A (standard), serum albumin (vitality), sodium chloride (USP)
Efficacy Effects
Temporary improvement in the appearance of moderate to severe glabella wrinkles (vertical lines between the eyebrows) associated with corrugator muscle and/or procerus muscle activities, in adults aged between 20 to 65.
Storage Methods
Sealed container, refrigerated (2 ~ 8 C) Storage
Date of Use
36 months from date of manufacture
Product Advantages
Resume normal work right away
Just by few tiny injections
Effects visible after one administration, starting from 2 weeks after injection, and peaks around 3~4 weeks.
Painless even when used without aenesthetic
Effects last for 6~12 months
Approved by Health Authorities with over 8 years’ clinical practices.
Rug Effects
After Injection
There is the possibility of reactions at the site of an injection, and effects beyond the injection site, including but not limited to asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. Serious hypersensitivity has been reported as rare.
There is a caution for people with pre-existing neuromuscular disorders, and for those with corneal exposure and ulceration in blepharospasm.
Botulium Toxin Type A is not to be administered at all if a patient has a known allergy to any of the ingredients, or if the patients have neuromuscular junctional disorders or known to have adverse reactions.
To preserve results achieved after a treatment with this Botulinum Toxin Type A injectable, patients need to undergo maintenance injection procedure
Other Products
 Hipretty PDO Thread |  Younsofill Dermal Fillers |
 Cindella + Luthione + VitaminC |  Lipolab |  LIporase |